6.1: Число крота та Авогадро
- Page ID
- 21746
- Розрахуйте формульні маси для ковалентних і іонних сполук.
- Визначте суму одиниці моль і пов'язану кількість числа Авогадро.
- Обчисліть молярну масу сполуки за молекулярною формулою.
Можна стверджувати, що сучасна хімічна наука почалася тоді, коли вчені почали досліджувати як кількісні, так і якісні аспекти хімії. Наприклад, атомна теорія Дальтона була спробою пояснити результати вимірювань, що дозволило йому обчислити відносні маси елементів, об'єднаних в різні сполуки. Розуміння взаємозв'язку між масами атомів і хімічними формулами сполук дозволяє кількісно описати склад речовин.
Формула маси
У попередньому розділі ми описали розвиток одиниці атомної маси, поняття середніх атомних мас та використання хімічних формул для представлення елементарного складу речовин. Ці ідеї можна розширити, щоб обчислити формулу маси речовини, яка дорівнює сумі атомних мас для всіх атомів, представлених у формулі речовини.
Формула маси для ковалентних речовин
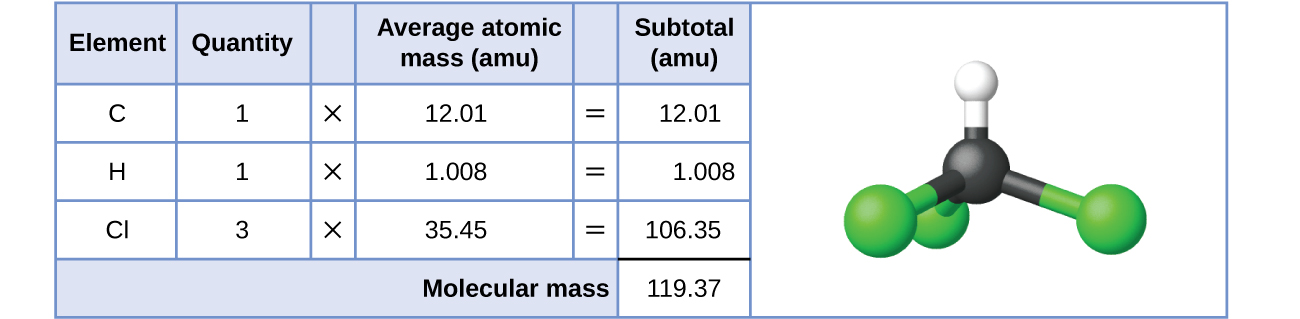
Для ковалентних речовин формула представляє числа та типи атомів, що складають одну молекулу речовини; отже, масу формули можна правильно назвати молекулярною масою. Розглянемо хлороформ (ChCl 3), ковалентне з'єднання, яке колись використовувалося в якості хірургічного анестетика і тепер в основному використовується у виробництві тетрафторетилену, будівельного блоку для «антипригарного» полімеру, тефлону. Молекулярна формула хлороформу вказує на те, що одна молекула містить один атом вуглецю, один атом водню і три атома хлору. Отже, середня молекулярна маса молекули хлороформу дорівнює сумі середніх атомних мас цих атомів. На\(\PageIndex{1}\) малюнку наводяться розрахунки, використані для виведення молекулярної маси хлороформу, яка становить 119.37 amu.
Так само молекулярна маса молекули аспірину, C 9 H 8 O 4, є сумою атомних мас дев'яти атомів вуглецю, восьми атомів водню та чотирьох атомів кисню, що становить 180,15 аму (рис.\(\PageIndex{2}\)).
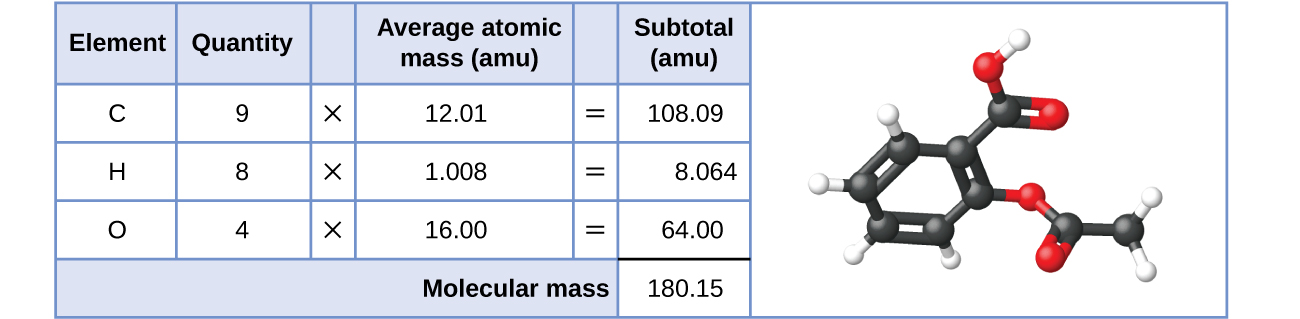
 Малюнок\(\PageIndex{2}\): The average mass of an aspirin molecule is 180.15 amu. The model shows the molecular structure of aspirin, C9H8O4.
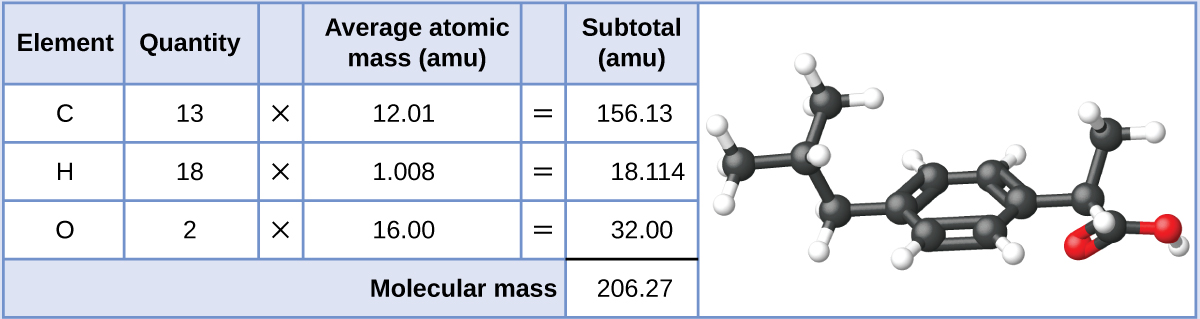
Малюнок\(\PageIndex{2}\): The average mass of an aspirin molecule is 180.15 amu. The model shows the molecular structure of aspirin, C9H8O4.Ibuprofen, C13H18O2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Advil and Motrin. What is the molecular mass (amu) for this compound?
Solution
Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. Following the approach described above, the average molecular mass for this compound is therefore:
Acetaminophen, C8H9NO2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Tylenol. What is the molecular mass (amu) for this compound?
- Answer
-
151.16 amu
Formula Mass for Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compound’s formula. Keep in mind, however, that the formula for an ionic compound does not represent the composition of a discrete molecule, so it may not correctly be referred to as the “molecular mass.”
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl−, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (Figure \(\PageIndex{3}\)).
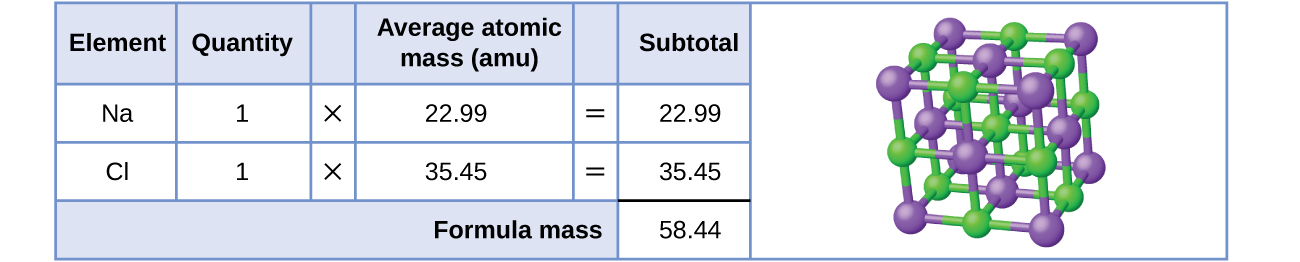
Note that the average masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. This approach is perfectly acceptable when computing the formula mass of an ionic compound. Even though a sodium cation has a slightly smaller mass than a sodium atom (since it is missing an electron), this difference will be offset by the fact that a chloride anion is slightly more massive than a chloride atom (due to the extra electron). Moreover, the mass of an electron is negligibly small with respect to the mass of a typical atom. Even when calculating the mass of an isolated ion, the missing or additional electrons can generally be ignored, since their contribution to the overall mass is negligible, reflected only in the nonsignificant digits that will be lost when the computed mass is properly rounded. The few exceptions to this guideline are very light ions derived from elements with precisely known atomic masses.
Aluminum sulfate, Al2(SO4)3, is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the formula mass (amu) of this compound?
Solution
The formula for this compound indicates it contains Al3+ and SO42− ions combined in a 2:3 ratio. For purposes of computing a formula mass, it is helpful to rewrite the formula in the simpler format, Al2S3O12. Following the approach outlined above, the formula mass for this compound is calculated as follows:

Calcium phosphate, \(\ce{Ca3(PO4)2}\), is an ionic compound and a common anti-caking agent added to food products. What is the formula mass (amu) of calcium phosphate?
- Answer
-
310.18 amu
The Mole
So far, we have been talking about chemical substances in terms of individual atoms and molecules. Yet we do not typically deal with substances one atom or molecule at a time; we work with millions, billions, and trillions of atoms and molecules at a time. We need a way to deal with macroscopic, rather than microscopic, amounts of matter. We need a unit of amount that relates quantities of substances on a scale that we can interact with.
Chemistry uses a unit called mole. The mole (mol) is an counting term similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities (such as atoms, molecules, and ions) as the number of atoms in a sample of pure 12C weighing exactly 12 g. One Latin connotation for the word “mole” is “large mass” or “bulk,” which is consistent with its use as the name for this unit. The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth.
The number of entities composing a mole has been experimentally determined to be \(6.02214179 \times 10^{23}\), a fundamental constant named Avogadro’s number (\(N_A\)) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of “per mole,” a conveniently rounded version being \(6.022 \times 10^{23}/\ce{mol}\).
How big is a mole? It is very large. Suppose you had a mole of dollar bills that need to be counted. If everyone on earth (about 6 billion people) counted one bill per second, it would take about 3.2 million years to count all the bills. A mole of sand would fill a cube about 32 km on a side. A mole of pennies stacked on top of each other would have about the same diameter as our galaxy, the Milky Way. Atoms and molecules are very tiny, so one mole of carbon atoms would make a cube that is 1.74 cm on a side, small enough to carry in your pocket. One mole of water molecules is approximately 18 mL or just under 4 teaspoons of water.
How many molecules are present in 2.76 mol of H2O? How many atoms is this?
Solution
The definition of a mole is an equality that can be used to construct a conversion factor. Also, because we know that there are three atoms in each molecule of H2O, we can also determine the number of atoms in the sample.
\[2.76\, \cancel{mol\, H_{2}O}\times \frac{6.022\times 10^{23}molecules\, H_{2}O}{\cancel{mol\, H_{2}O}}=1.66\times 10^{24}molecules\, H_{2}O \nonumber\nonumber \]
To determine the total number of atoms, we have
\[1.66\times 10^{24}\cancel{molecules\, H_{2}O}\times \frac{3\, atoms}{1\, molecule}=4.99\times 10^{24}\, atoms \nonumber\nonumber \]
How many molecules are present in 4.61 × 10−2 mol of \(\ce{O2}\)?
- Answer
-
2.78 × 1022 molecules
Molar Mass
Why is the mole unit so important? It represents the link between the microscopic and the macroscopic, especially in terms of mass. A mole of a substance has the same mass in grams as one unit (atom or molecules) has in atomic mass units. The mole unit allows us to express amounts of atoms and molecules in visible amounts that we can understand.
For example, we already know that, by definition, a mole of carbon has a mass of exactly 12 g. This means that exactly 12 g of C has 6.022 × 1023 atoms:
12 g C = 6.022 × 1023 atoms C
We can use this equality as a conversion factor between the number of atoms of carbon and the number of grams of carbon. How many grams are there, say, in 1.50 × 1025 atoms of carbon? This is a one-step conversion:
\[1.50\times 10^{25}\cancel{atoms\, C}\times \frac{12.0000\, g\, C}{6.022\times 10^{23}\cancel{atoms\, C}}=299\, g\, C\nonumber \]
But it also goes beyond carbon. Previously we defined atomic and molecular masses as the number of atomic mass units per atom or molecule. Now we can do so in terms of grams. The atomic mass of an element is the number of grams in 1 mol of atoms of that element, while the molecular mass of a compound is the number of grams in 1 mol of molecules of that compound. Sometimes these masses are called molar masses to emphasize the fact that they are the mass for 1 mol of things. (The term molar is the adjective form of mole and has nothing to do with teeth.)
Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (Figure \(\PageIndex{1}\)).

Because the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. Per the amu definition, a single 12C atom weighs 12 amu (its atomic mass is 12 amu). According to the definition of the mole, 12 g of 12C contains 1 mole of 12C atoms (its molar mass is 12 g/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C.
| Element | Average Atomic Mass (amu) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | \(6.022 \times 10^{23}\) |
| H | 1.008 | 1.008 | \(6.022 \times 10^{23}\) |
| O | 16.00 | 16.00 | \(6.022 \times 10^{23}\) |
| Na | 22.99 | 22.99 | \(6.022 \times 10^{23}\) |
| Cl | 33.45 | 35.45 | \(6.022 \times 10^{23}\) |
While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units (amu versus g). To appreciate the enormity of the mole, consider a small drop of water after a rainfall. Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules.
The relationships between formula mass, the mole, and Avogadro’s number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass.
Here are some examples. The mass of 1 hydrogen atom is 1.0079 u; the mass of 1 mol of hydrogen atoms is 1.0079 g. Elemental hydrogen exists as a diatomic molecule, H2. One molecule has a mass of 1.0079 u + 1.0079 u = 2.0158 u, while 1 mol of H2 has a mass of 1.0079 g + 1.0079 g = 2.0158 g. One molecule of H2O has a mass of about 18.01 u; 1 mol H2O has a mass of 18.01 g. A single unit of NaCl has a mass of 58.45 u; NaCl has a molar mass of 58.45 g. In each of these moles of substances, there are 6.022 × 1023 units: 6.022 × 1023 atoms of H, 6.022 × 1023 molecules of H2 and H2O, 6.022 × 1023 units of NaCl ions. These relationships give us plenty of opportunities to construct conversion factors for simple calculations.
Sugar
What is the molar mass of sugar (\(\ce{C6H12O6}\))?
Solution
To determine the molar mass, we simply add the atomic masses of the atoms in the molecular formula; but express the total in grams per mole, not atomic mass units. The masses of the atoms can be taken from the periodic table.
| 6 C = 6 × 12.011 | = 72.066 |
|---|---|
| 12 H = 12 × 1.0079 | = 12.0948 |
| 6 O = 6 × 15.999 | = 95.994 |
| TOTAL | = 180.155 g/mol |
Per convention, the unit grams per mole is written as a fraction.
What is the molar mass of \(\ce{AgNO3}\)?
- Answer
-
169.87 g/mol
Summary
The mole is a key unit in chemistry. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's mass in atomic mass units.