10.4: Гума та інші еластомери
- Page ID
- 20315
- Знати властивості гуми.
- Опишіть процес вулканізації.
Натуральний каучук, також званий індійським каучуком або каучуком, як спочатку виробляється, складається з полімерів органічної сполуки ізопрену, з незначними домішками інших органічних сполук, плюс вода. Таїланд і Індонезія є двома провідними виробниками гуми. Форми поліізопрену, які використовуються в якості натуральних каучуків, класифікуються як еластомери.
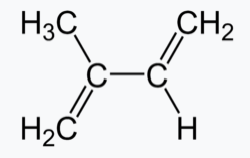
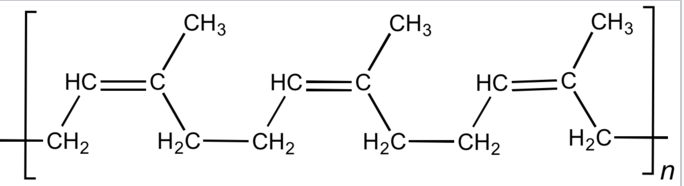
Ізопрен Поліізопрен (каучук)
В даний час гуму заготовляють переважно у вигляді латексу з каучукового дерева або інших. Латекс - це липкий молочний колоїд, витягнутий, роблячи надрізи в корі і збираючи рідину в судині в процесі, який називається «постукування». Потім латекс переробляється в гуму, готову до комерційної переробки. У основних областях латексу дозволяється коагулювати в чашці для збору. Згорнуті грудочки збирають і переробляють в сухі форми для збуту.
Натуральний каучук широко використовується в багатьох додатках і продуктах, окремо або в поєднанні з іншими матеріалами. У більшості своїх корисних форм він має великий коефіцієнт розтягування та високу стійкість, а також надзвичайно водонепроникний.
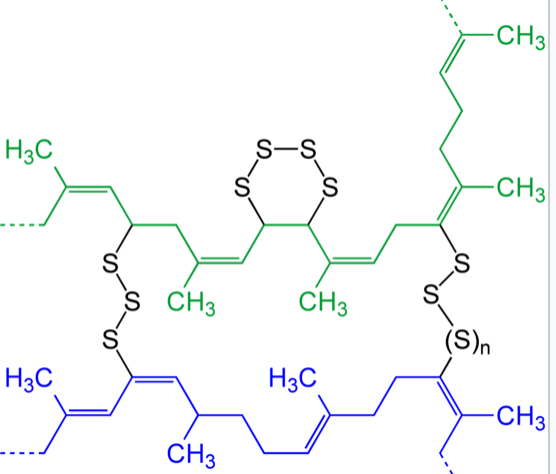
Вулканізація
In 1832–1834 Nathaniel Hayward and Friedrich Ludersdorf discovered that rubber treated with sulfur lost its stickiness. It is likely Hayward shared his discovery with Charles Goodyear, possibly inspiring him to make the discovery of vulcanization. Thomas Hancock (1786–1865), a scientist and engineer, was the first to patent vulcanization of rubber. He was awarded a British patent on May 21, 1845. Three weeks later, on June 15, 1845, Charles Goodyear was awarded a patent in the United States. It was Hancock's friend William Brockedon who coined term 'vulcanization'. Goodyear claimed that he had discovered vulcanization earlier, in 1839.
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into more durable materials by heating them with sulfur or other equivalent curatives or accelerators. Sulfur forms cross-links (bridges) between sections of polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electronic properties of the material. A vast array of products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts. The term vulcanization is derived from Vulcan, the Roman god of fire.
Synthetic Rubber
The expanded use of bicycles, and particularly their pneumatic tires, starting in the 1880s, created increased demand for rubber. In 1909 a team headed by Fritz Hofmann, working at the Bayer laboratory in Germany, succeeded in polymerizing isoprene, the first synthetic rubber. A synthetic rubber is any artifiical elastomer. These are mainly polymers synthesized from petroleum by products.
Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production. Another 25% is used as an additive to improve the toughness (impact resistance) of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS). Polybutadiene rubber accounted for about a quarter of total global consumption of synthetic rubbers in 2012. It is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity.
Neoprene (also polychloroprene or pc-rubber) is a family of synthetic rubbers that are produced by polymerization of chloroprene. Neoprene exhibits good chemical stability and maintains flexibility over a wide temperature range. Neoprene is sold either as solid rubber or in latex form and is used in a wide variety of applications, such as laptop sleeves, orthopaedic braces (wrist, knee, etc.), electrical insulation, liquid and sheet applied elastomeric membranes or flashings, and automotive fan belts.Neoprene is produced by free-radical polymerization of chloroprene. In commercial production, this polymer is prepared by free radical emulsion polymerization. Polymerization is initiated using potassium persulfate. Bifunctional nucleophiles, metal oxides (e.g. zinc oxide), and thioureas are used to crosslink individual polymer strands.
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR.
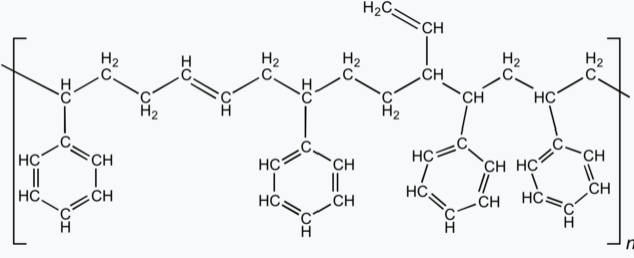
It is a commodity material which competes with natural rubber. The elastomer is used widely in pneumatic tires. Other uses include shoe heels and soles, gaskets, and even chewing gum.
Polymers in Paints
Polymers are one of the key components of modern paints that function as binders. The binder is the film-forming component of paint. It is the only component that is always present among all the various types of formulations. The binder imparts properties such as gloss, durability, flexibility, and toughness. Binders include synthetic or natural resins such as alkyds, acrylics, vinyl-acrylics, vinyl acetate/ethylene (VAE), polyurethanes, polyesters, melamine resins, epoxy, or siloxanes or oils.
Summary
The many uses of natural rubber has led to development and manufacture of synthetic rubber.
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into more durable materials by heating them with sulfur or other equivalent curatives or accelerators.
Three examples of synthetic rubber used in various applications are polybutadiene, polychloroprene (Neoprene), and styrene-butadiene rubber (SBR)
